|
· Home
· Research
· Members
· Publications
· Photos
· MAGMUN
· SQUID
|
Research
Interests
High Nuclearity
Grids and Clusters (M2 to M36 and Beyond)
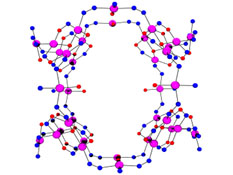 Strategies to produce
coordination complexes with large numbers of transition metal centers include
direct synthesis from a polyfunctional ligand, and methods which use Strategies to produce
coordination complexes with large numbers of transition metal centers include
direct synthesis from a polyfunctional ligand, and methods which use 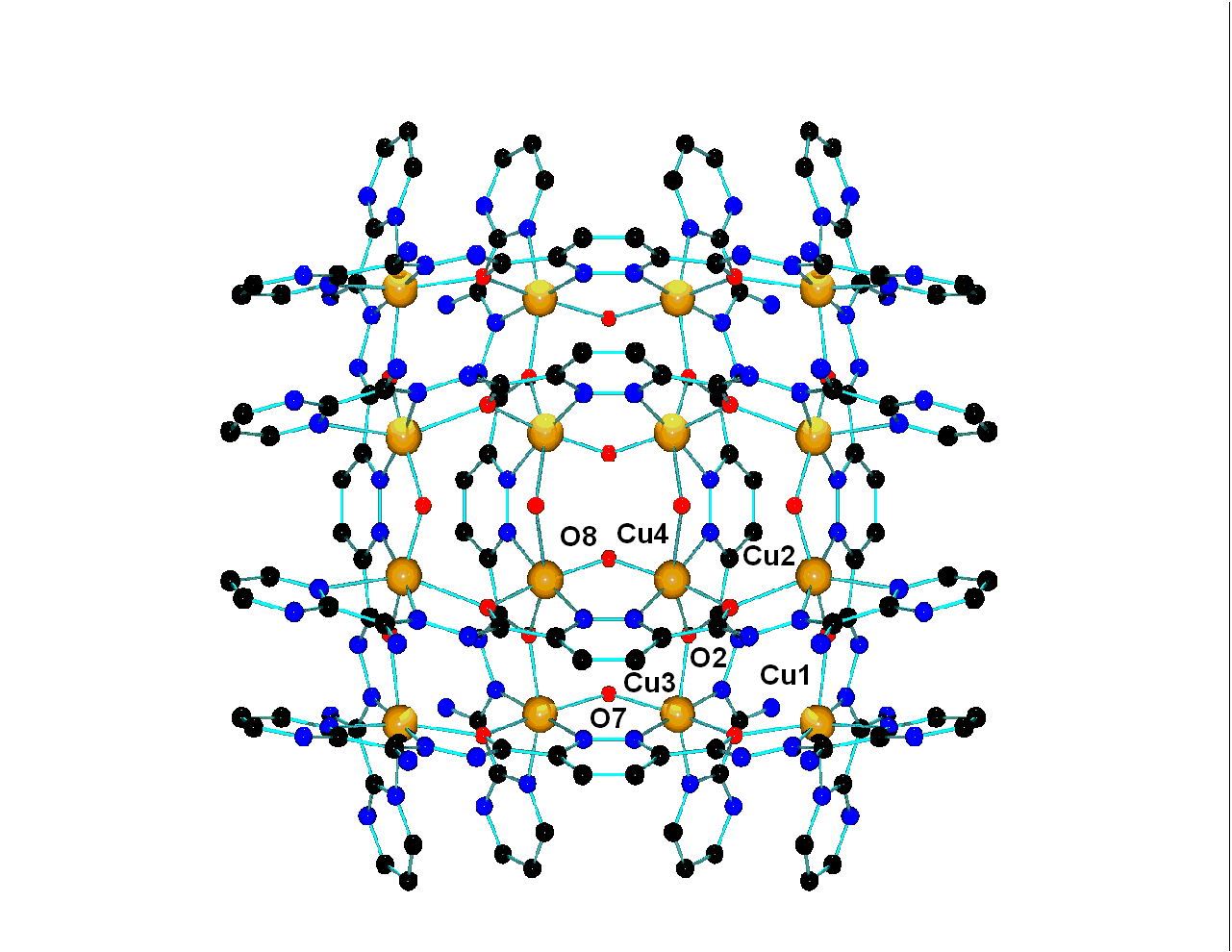 the organizing ability of a
metal ion and a ligand or a ligand precursor (e.g. template syntheses). Self
assembly is a powerful approach which involves the encoding of coordination
information into a ligand, and then using a metal ion to interpret and use
this information, according to its own coordination preferences, in order to
organize the growth of large polynuclear metal ion arrays. These are of
interest from a variety of viewpoints, including their inherent beauty, but
more practically because they provide routes to novel magnetic materials,
potential catalysts, and in the context of a ‘bottom up’ approach to
‘devices’ based on molecules, an entry into the electronic, and perhaps
magnetic, high technology arena of the future. Grid arrangements of
transition metal ions bridged in close proximity are viewed as ‘quantum dot’
like arrays of communicating spin centers, and are very attractive platforms
for switching and data storage at the molecular level. Our current research
projects in this area involve studies on square [nxn] grids (n=2, 3, 4, 5), with
magnetically and electrochemically active metal ions, e.g. Mn(II), Fe(II),
Fe(III), Co(II), Ni(II), Cu(II). Such systems are obtained by self assembly
synthetic strategies, and studied using structural, magnetic,
electrochemical, and epr techniques. Strategies for larger [nxn] grids (n =
6, 8) are being developed with hexa- and octa-topic ligands as targets. the organizing ability of a
metal ion and a ligand or a ligand precursor (e.g. template syntheses). Self
assembly is a powerful approach which involves the encoding of coordination
information into a ligand, and then using a metal ion to interpret and use
this information, according to its own coordination preferences, in order to
organize the growth of large polynuclear metal ion arrays. These are of
interest from a variety of viewpoints, including their inherent beauty, but
more practically because they provide routes to novel magnetic materials,
potential catalysts, and in the context of a ‘bottom up’ approach to
‘devices’ based on molecules, an entry into the electronic, and perhaps
magnetic, high technology arena of the future. Grid arrangements of
transition metal ions bridged in close proximity are viewed as ‘quantum dot’
like arrays of communicating spin centers, and are very attractive platforms
for switching and data storage at the molecular level. Our current research
projects in this area involve studies on square [nxn] grids (n=2, 3, 4, 5), with
magnetically and electrochemically active metal ions, e.g. Mn(II), Fe(II),
Fe(III), Co(II), Ni(II), Cu(II). Such systems are obtained by self assembly
synthetic strategies, and studied using structural, magnetic,
electrochemical, and epr techniques. Strategies for larger [nxn] grids (n =
6, 8) are being developed with hexa- and octa-topic ligands as targets.
Ligand Syntheses
The success of creating polynuclear complexes by either direct synthesis
or a self-assembly approach relies heavily on the use of organic synthetic
techniques to produce new ligands and ligand precursors. This has been the
cornerstone of our success in the polynuclear complex area for many years,
and we continually strive to create new and novel ligand systems, which will
provide a ‘directed’ synthetic approach to high nuclearity systems. Fine
tuning of the ligand in terms of the donor atoms and their spatial
arrangements has allowed us to create many classes of ligand which, with
experience, can produce predictable polynuclear complexes, particularly
self-assembled grids. Organic synthesis continues to be a vital part of our
approach to new and novel materials.
Magnetic Studies
Our interest in the creation of polynuclear complexes has been driven in
part by our deep seated interest in the magnetic properties of such systems,
and in particular the geometrical and electronic properties of the ligands as
they pertain to magnetic properties at the molecular level based on the
transition metal ion. We have published widely on magneto-structural
correlations in e.g. dinuclear 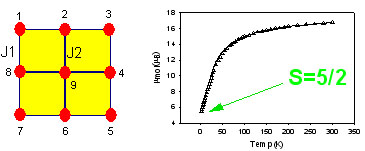 complexes
involving hydroxide, phenoxide, and 1,1-azide bridges, and more recently have
been examining high nuclearity systems, in particular [nxn] grid arrays of metal
ions bridged in close proximity by oxygen atoms, e.g. [M9(µ-O)12].
These systems display antiferromagnetic (Mn(II), Fe(II), Fe(III), Ni(II),
Co(II)) and ferromagnetic (Cu(II)) behavior, with the Mn(II) grids acting as
quantum nano-magnets at low temperature. Dealing with large numbers of spin
centers in a single molecule presents special computing challenges, because
of the large matrix dimensions involved in calculating the total spin state
combinations. We have developed an integrated software package (MAGMUN4.0),
built on a Windows™ platform, which handles large systems (e.g. Co(II)9;
(S=3/2)9) successfully, and are in the process of implementing
symmetry reduction methods to handle larger systems. As an illustration a
high spin Mn(II)9 grid would require ~60 GB of RAM for a full
isotropic spin state calculation, which can be reduced to ~ 4GB by imposing D4
symmetry on the grid. complexes
involving hydroxide, phenoxide, and 1,1-azide bridges, and more recently have
been examining high nuclearity systems, in particular [nxn] grid arrays of metal
ions bridged in close proximity by oxygen atoms, e.g. [M9(µ-O)12].
These systems display antiferromagnetic (Mn(II), Fe(II), Fe(III), Ni(II),
Co(II)) and ferromagnetic (Cu(II)) behavior, with the Mn(II) grids acting as
quantum nano-magnets at low temperature. Dealing with large numbers of spin
centers in a single molecule presents special computing challenges, because
of the large matrix dimensions involved in calculating the total spin state
combinations. We have developed an integrated software package (MAGMUN4.0),
built on a Windows™ platform, which handles large systems (e.g. Co(II)9;
(S=3/2)9) successfully, and are in the process of implementing
symmetry reduction methods to handle larger systems. As an illustration a
high spin Mn(II)9 grid would require ~60 GB of RAM for a full
isotropic spin state calculation, which can be reduced to ~ 4GB by imposing D4
symmetry on the grid.
Electrochemical
and Surface Studies
The Mn(II)9 grids exhibit very rich electrochemistry, with the transfer
of up to eight electrons within the potential window 0.5-1.6 V (Ag/AgCl),
associated with the oxidation of eight Mn(II) centers to Mn(III). Oxidation
can also be achieved using chemical oxidants. The magnetic and spectroscopic
properties of the grids change on oxidation, which can be detected easily
using SQUID measurements and UV/Vis spectroscopy. These processes are
reversible both electrochemically and chemically, and so the grids display
multi-property function. A major current focus is to try to detect a single
molecule response based on these bulk properties, and an obvious way to try
this is to first immobilize the molecules on a suitable surface substrate.
This has been achieved in several cases on Au(111), with the formation of
self-assembled monolayers (SAMS) and a current thrust is to probe individual
molecules on the surface electrochemically and by single molecule epr
techniques.
Device
Applications
 The micro-electronics
industry is still operating on a ‘top-down’ principle, which in many areas,
e.g. computers, is leading to serious limitations regarding processing speed,
and data storage capacity. The ‘bottom-up’ approach, using atoms or
molecules, is seen as the way of the future, but harnessing the power of an
individual molecule is a major challenge. We have demonstrated multi-electron
reversibility in the Mn(II) grid systems, and this property is being
exploited at the molecular level in terms of ‘molecular device behavior’.
Such studies require overcoming obstacles not normally encountered in simple
bench chemistry, which present rather different challenges. In this context
we have established collaborations with physicists and surface chemists to
assist us in probing these systems at the molecular level. As a simple
illustration SAMS of Mn(II)9 grid molecules, which have an
~2.5x2.5 nm footprint on Au(111), would be capable of storing of the order of
100-150 Tb/in2 with one bit of encoded information per molecule. The micro-electronics
industry is still operating on a ‘top-down’ principle, which in many areas,
e.g. computers, is leading to serious limitations regarding processing speed,
and data storage capacity. The ‘bottom-up’ approach, using atoms or
molecules, is seen as the way of the future, but harnessing the power of an
individual molecule is a major challenge. We have demonstrated multi-electron
reversibility in the Mn(II) grid systems, and this property is being
exploited at the molecular level in terms of ‘molecular device behavior’.
Such studies require overcoming obstacles not normally encountered in simple
bench chemistry, which present rather different challenges. In this context
we have established collaborations with physicists and surface chemists to
assist us in probing these systems at the molecular level. As a simple
illustration SAMS of Mn(II)9 grid molecules, which have an
~2.5x2.5 nm footprint on Au(111), would be capable of storing of the order of
100-150 Tb/in2 with one bit of encoded information per molecule.
|
 Strategies to produce
coordination complexes with large numbers of transition metal centers include
direct synthesis from a polyfunctional ligand, and methods which use
Strategies to produce
coordination complexes with large numbers of transition metal centers include
direct synthesis from a polyfunctional ligand, and methods which use  the organizing ability of a
metal ion and a ligand or a ligand precursor (e.g. template syntheses). Self
assembly is a powerful approach which involves the encoding of coordination
information into a ligand, and then using a metal ion to interpret and use
this information, according to its own coordination preferences, in order to
organize the growth of large polynuclear metal ion arrays. These are of
interest from a variety of viewpoints, including their inherent beauty, but
more practically because they provide routes to novel magnetic materials,
potential catalysts, and in the context of a ‘bottom up’ approach to
‘devices’ based on molecules, an entry into the electronic, and perhaps
magnetic, high technology arena of the future. Grid arrangements of
transition metal ions bridged in close proximity are viewed as ‘quantum dot’
like arrays of communicating spin centers, and are very attractive platforms
for switching and data storage at the molecular level. Our current research
projects in this area involve studies on square [nxn] grids (n=2, 3, 4, 5), with
magnetically and electrochemically active metal ions, e.g. Mn(II), Fe(II),
Fe(III), Co(II), Ni(II), Cu(II). Such systems are obtained by self assembly
synthetic strategies, and studied using structural, magnetic,
electrochemical, and epr techniques. Strategies for larger [nxn] grids (n =
6, 8) are being developed with hexa- and octa-topic ligands as targets.
the organizing ability of a
metal ion and a ligand or a ligand precursor (e.g. template syntheses). Self
assembly is a powerful approach which involves the encoding of coordination
information into a ligand, and then using a metal ion to interpret and use
this information, according to its own coordination preferences, in order to
organize the growth of large polynuclear metal ion arrays. These are of
interest from a variety of viewpoints, including their inherent beauty, but
more practically because they provide routes to novel magnetic materials,
potential catalysts, and in the context of a ‘bottom up’ approach to
‘devices’ based on molecules, an entry into the electronic, and perhaps
magnetic, high technology arena of the future. Grid arrangements of
transition metal ions bridged in close proximity are viewed as ‘quantum dot’
like arrays of communicating spin centers, and are very attractive platforms
for switching and data storage at the molecular level. Our current research
projects in this area involve studies on square [nxn] grids (n=2, 3, 4, 5), with
magnetically and electrochemically active metal ions, e.g. Mn(II), Fe(II),
Fe(III), Co(II), Ni(II), Cu(II). Such systems are obtained by self assembly
synthetic strategies, and studied using structural, magnetic,
electrochemical, and epr techniques. Strategies for larger [nxn] grids (n =
6, 8) are being developed with hexa- and octa-topic ligands as targets.  complexes
involving hydroxide, phenoxide, and 1,1-azide bridges, and more recently have
been examining high nuclearity systems, in particular [nxn] grid arrays of metal
ions bridged in close proximity by oxygen atoms, e.g. [M9(µ-O)12].
These systems display antiferromagnetic (Mn(II), Fe(II), Fe(III), Ni(II),
Co(II)) and ferromagnetic (Cu(II)) behavior, with the Mn(II) grids acting as
quantum nano-magnets at low temperature. Dealing with large numbers of spin
centers in a single molecule presents special computing challenges, because
of the large matrix dimensions involved in calculating the total spin state
combinations. We have developed an integrated software package (MAGMUN4.0),
built on a Windows™ platform, which handles large systems (e.g. Co(II)9;
(S=3/2)9) successfully, and are in the process of implementing
symmetry reduction methods to handle larger systems. As an illustration a
high spin Mn(II)9 grid would require ~60 GB of RAM for a full
isotropic spin state calculation, which can be reduced to ~ 4GB by imposing D4
symmetry on the grid.
complexes
involving hydroxide, phenoxide, and 1,1-azide bridges, and more recently have
been examining high nuclearity systems, in particular [nxn] grid arrays of metal
ions bridged in close proximity by oxygen atoms, e.g. [M9(µ-O)12].
These systems display antiferromagnetic (Mn(II), Fe(II), Fe(III), Ni(II),
Co(II)) and ferromagnetic (Cu(II)) behavior, with the Mn(II) grids acting as
quantum nano-magnets at low temperature. Dealing with large numbers of spin
centers in a single molecule presents special computing challenges, because
of the large matrix dimensions involved in calculating the total spin state
combinations. We have developed an integrated software package (MAGMUN4.0),
built on a Windows™ platform, which handles large systems (e.g. Co(II)9;
(S=3/2)9) successfully, and are in the process of implementing
symmetry reduction methods to handle larger systems. As an illustration a
high spin Mn(II)9 grid would require ~60 GB of RAM for a full
isotropic spin state calculation, which can be reduced to ~ 4GB by imposing D4
symmetry on the grid.  The micro-electronics
industry is still operating on a ‘top-down’ principle, which in many areas,
e.g. computers, is leading to serious limitations regarding processing speed,
and data storage capacity. The ‘bottom-up’ approach, using atoms or
molecules, is seen as the way of the future, but harnessing the power of an
individual molecule is a major challenge. We have demonstrated multi-electron
reversibility in the Mn(II) grid systems, and this property is being
exploited at the molecular level in terms of ‘molecular device behavior’.
Such studies require overcoming obstacles not normally encountered in simple
bench chemistry, which present rather different challenges. In this context
we have established collaborations with physicists and surface chemists to
assist us in probing these systems at the molecular level. As a simple
illustration SAMS of Mn(II)9 grid molecules, which have an
~2.5x2.5 nm footprint on Au(111), would be capable of storing of the order of
100-150 Tb/in2 with one bit of encoded information per molecule.
The micro-electronics
industry is still operating on a ‘top-down’ principle, which in many areas,
e.g. computers, is leading to serious limitations regarding processing speed,
and data storage capacity. The ‘bottom-up’ approach, using atoms or
molecules, is seen as the way of the future, but harnessing the power of an
individual molecule is a major challenge. We have demonstrated multi-electron
reversibility in the Mn(II) grid systems, and this property is being
exploited at the molecular level in terms of ‘molecular device behavior’.
Such studies require overcoming obstacles not normally encountered in simple
bench chemistry, which present rather different challenges. In this context
we have established collaborations with physicists and surface chemists to
assist us in probing these systems at the molecular level. As a simple
illustration SAMS of Mn(II)9 grid molecules, which have an
~2.5x2.5 nm footprint on Au(111), would be capable of storing of the order of
100-150 Tb/in2 with one bit of encoded information per molecule.